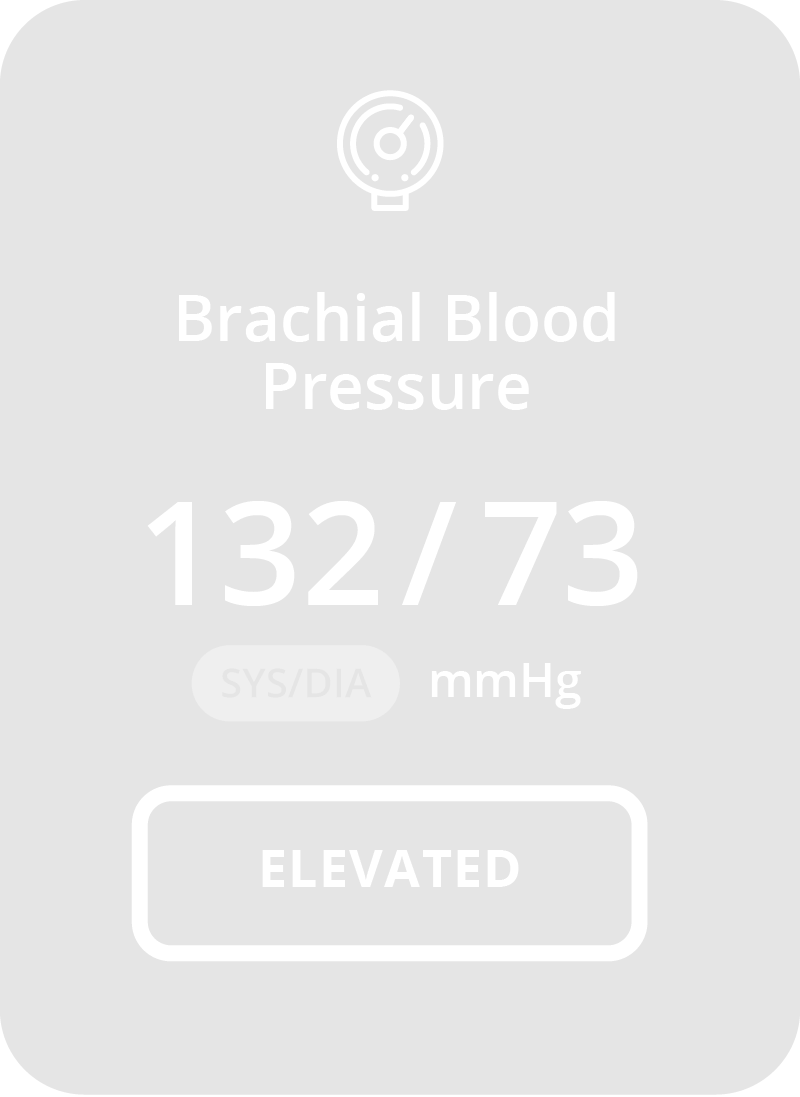
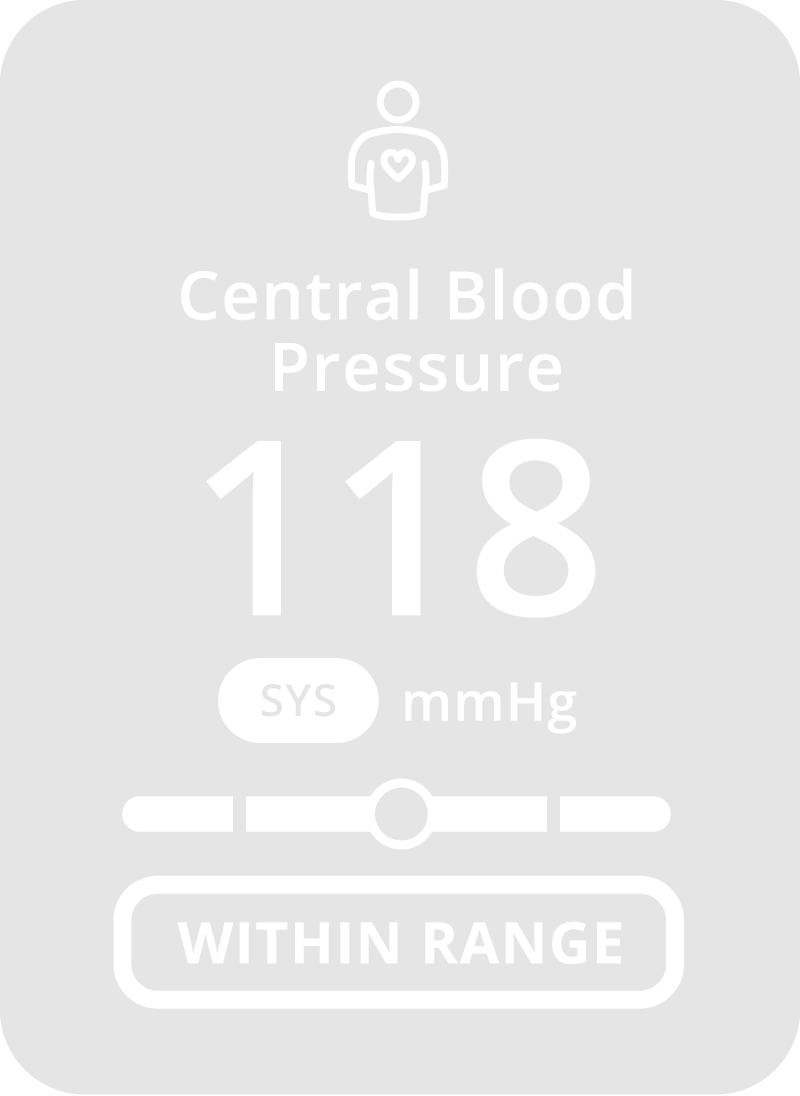
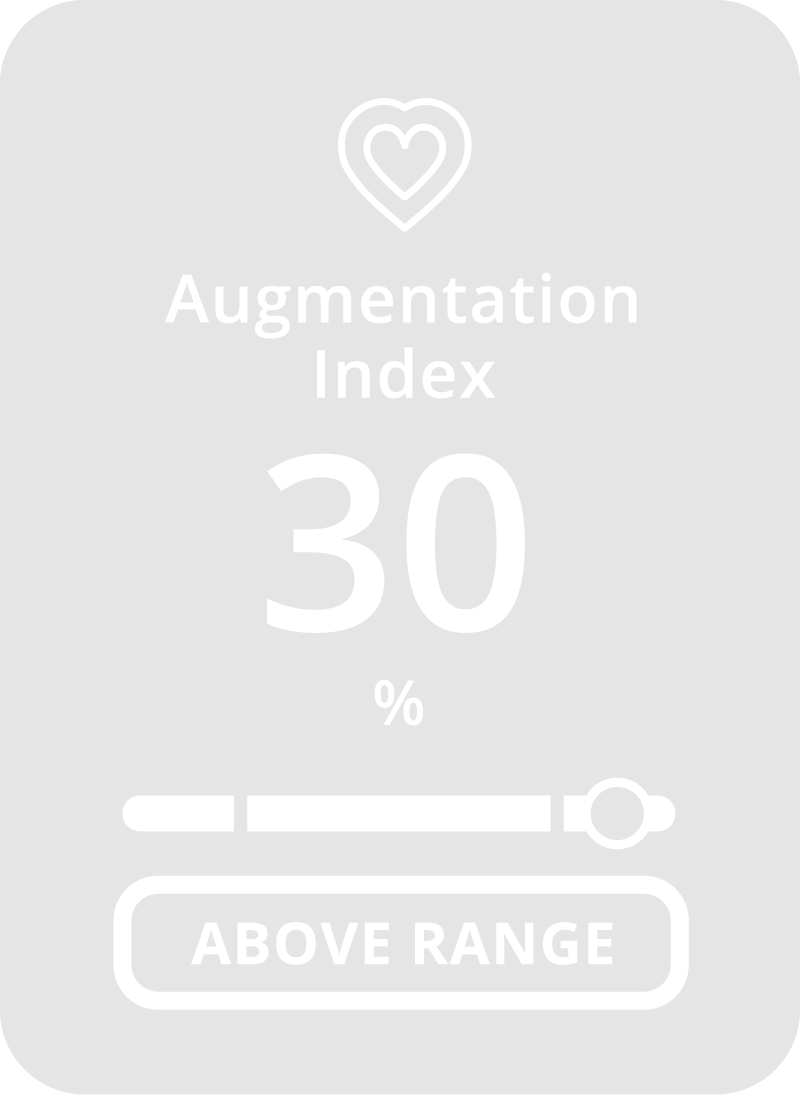
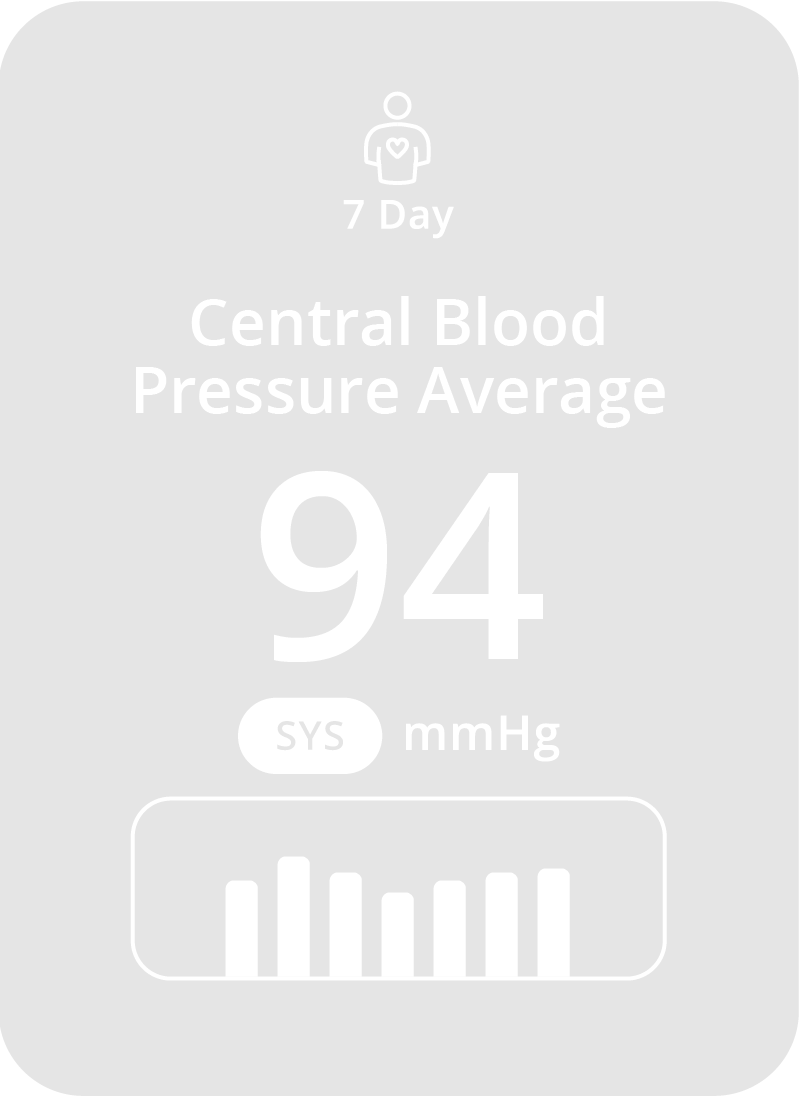
Why CONNEQT
The Clinical Standard for Arterial Health Assessment
Clinical-grade arterial insights, from clinic to home

Arterial health influences the heart, brain, kidneys, and healthspan as we age.
You want to support healthy aging.
You’re concerned how hypertension impacts your heart, kidneys, and brain.
You want a device backed by cardiologists, validated research, and clinical trials.
You want to track cardiovascular health over time at home.
You want insights that is clear, personalized, accurate, and clinically grounded.
You’re making lifestyle changes and want to understand how they affect heart health.
You’re ready to investigate heart health due to fatigue, circulation issues, or risk signs.
CONNEQT brings arterial health into focus wherever health decisions are made.
Powered by SphygmoCor®
SphygmoCor is the clinical reference technology for measuring central blood pressure and arterial function, used for decades in cardiovascular research, drug development, and clinical care.
Top 20
All “Top 20 Hospitals” use SphygmoCor technology to measure central blood pressure (cBP)
3,000+
SphygmoCor technology has supported studies that have resulted in over 3,000 peer-reviewed publications.
8/10
8 out of top 10 Pharma companies have used SphygmoCor technology in their clinical trials
11,000
Over 11,000 patients have been tested with SphygmoCor technology in pharmaceutical trials
Trusted by the World's Leading Experts in Arterial Health
Chosen for Everyday Use at Home
Ratings and reviews from verified purchasers, based on real at-home use.