Most health devices measure something. Very few measure it well enough to matter.
Modern health tech produces numbers that feel precise and look scientific. Step counts, heart rate estimates, body weight down to a decimal. But precision and accuracy are not the same thing. When you’re tracking slow changes in cardiovascular health, that difference matters. Only FDA-cleared medical devices are allowed to measure and interpret blood pressure in a clinically meaningful way. General wellness products are regulated to provide awareness and estimates, not medical interpretation.

Measure what matters
Save 20% on checkout with code VITALITY
This is where clinical validation comes in. Clinical validation means a device has been tested against gold-standard equipment used in hospitals and research labs and shown to measure physiological signals reliably across real people, not just ideal conditions.
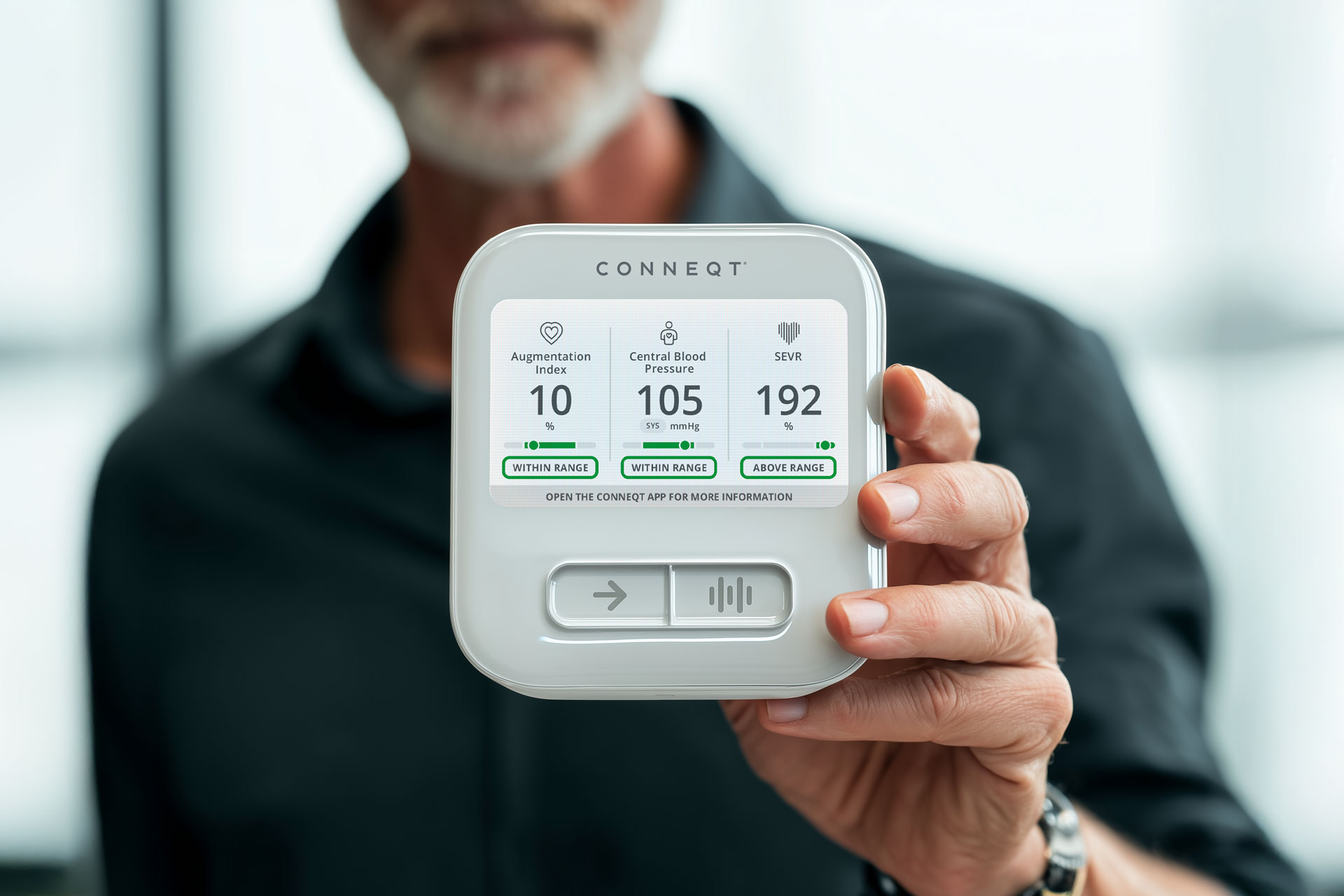
The CONNEQT Pulse is clinically validated. Here’s what that means in practice.
The Problem With “Close Enough”
Think about what you are actually trying to do with health data. You are looking for patterns. You want to know if your new sleep routine is working, if cutting back on alcohol is making a difference, or if your arteries are getting more flexible after months of exercise.
These changes happen gradually. They are subtle. And subtle signals are the first thing poor “good enough” measurement erases.
When a device consistently adds noise, exaggerates daily variation, or drifts over time, it becomes harder to tell whether changes are real. Meaningful improvements can be missed, while normal variation can appear more important than it actually is.
A clinically validated device doesn’t promise perfect readings every time. It offers something more valuable: measurements that reliably preserve the direction of change. When something shifts in your body, the data is designed to reflect that change over time.
What Clinical Validation Actually Tests
Clinical validation is not a marketing label. It is a defined and rigorous testing process that general “wellness products” (like wearables or health “rings”) do not go through.
In practice, a device’s measurements are compared directly against gold-standard clinical instruments used in hospitals, research labs, and large population studies with decades of published evidence behind them.
Many widely used wearables estimate cardiovascular signals using optical sensors worn on the wrist or finger. These approaches are designed for general lifestyle awareness rather than clinical measurement, and indirect estimation can make subtle arterial changes harder to detect over time.
Wearable products like rings and bands can provide helpful context and trend information, but blood pressure is a clinical measurement. To ensure accuracy, any blood pressure feature on a wearable should be checked against a clinically validated home arterial health monitor. Only FDA-cleared medical devices are allowed to confirm and interpret blood pressure in a clinically meaningful way.
Clinical validation goes further than a single comparison under ideal conditions. Validation protocols evaluate how a device performs across different body types, ages, and physiological states. The validation is demanding and time consuming in order to meet the standards of a “medical device”.
Wellness products tend to fall short of validation when they rely heavily on estimation instead of direct measurement, only perform well under ideal conditions, or show drift across repeated readings over time.
Medical devices pass validation when their measurements align with established clinical methods, remain stable across repeated readings, and reflect underlying physiology rather than compensating through software correction.
Why Arterial Signals Require Accurate Measurement
Traditional arm blood pressure cuffs measure pressure in the brachial artery, a large vessel close to the surface.
Arterial signals are different.
Measures such as central blood pressure, SEVR (subendocardial viability ratio), and indicators of arterial stiffness reflect how pressure moves through the arterial system itself. These signals depend on timing, wave reflection, and arterial elasticity.
That complexity is what makes them valuable. Arterial signals often change earlier, before symptoms appear and before brachial blood pressure rises into a diagnosable range. Measuring them well requires methods designed to capture subtle signals over time.
The Science Behind Arterial Measurement
Pulse wave analysis did not originate in consumer health. It emerged from decades of cardiovascular research aimed at understanding how arteries age and how pressure loads the heart and brain.
For decades, clinicians and researchers have relied on pulse wave analysis systems in major population studies and clinical trials examining arterial stiffness and central blood pressure, using standardized, research-grade methods.
The Pulse is built on that same underlying framework long used in clinical research. The principles and physics are unchanged. What differs is the ability to use them consistently at home, anchoring everyday measurements to the same frameworks used in clinical research.
What Validated Data Changes in Practice
With reliable measurements from the Pulse, daily fluctuations become easier to contextualize. Instead of reacting to every change, you can focus on trends that develop over weeks and months. You can evaluate habits with more confidence. Changes in sleep, stress, nutrition, or movement can be evaluated without guessing whether the signal is real.
You gain clarity around when to adjust and when to stay the course. Stable trends suggest consistency is working. Meaningful shifts signal that something in the system has changed. Because these measurements are FDA-cleared and clinically validated, they can also be used to generate structured reports and assessments that support clinician review and ongoing care, something general wellness products are not permitted to do.
Why Clean Signals Matter for Prevention
Preventive cardiovascular health relies on pattern recognition. That process breaks down when the signal is unreliable.
Clinical validation does not make health predictable. It makes data interpretable. It reduces guesswork and clarifies whether daily choices are influencing cardiovascular health.
The Pulse is not designed to diagnose disease or replace medical care. It is designed to provide FDA-cleared measurements, with Care+ supporting interpretation, grounded in decades of clinical research that reflect how your arteries respond over time.
When you are trying to detect change early, accuracy is not optional. It actually makes the data useful.